The Science of Balloons: Exploring the Physics and Chemistry Behind Inflation?
Deflated or burst balloons ruin perfect events and waste your budget. Why do some fail while others last? Understanding the science behind inflation saves you money and reputation.
Balloons inflate because gas particles create high internal pressure1, pushing against elastic polymer walls. This follows Boyle’s Law2. The quality of the latex links directly to how well these polymers stretch without breaking, ensuring your inventory stays inflated longer.
I have seen many buyers struggle with inconsistent quality because they overlook the basic science. We need to look closer at the details to understand why this happens.
How Does Latex Chemical Composition Affect Balloon Elasticity and Burst Resistance?
Cheap latex snaps easily, causing customer complaints and safety risks. You need materials that withstand real-world use without failing unexpectedly.
High-quality latex contains long, un-branched polymer chains. When we cure these with sulfur (vulcanization3), they form cross-links4. This chemical structure allows the balloon to stretch significantly under pressure and return to shape, resisting bursts better than fillers or cheap synthetic mixes.
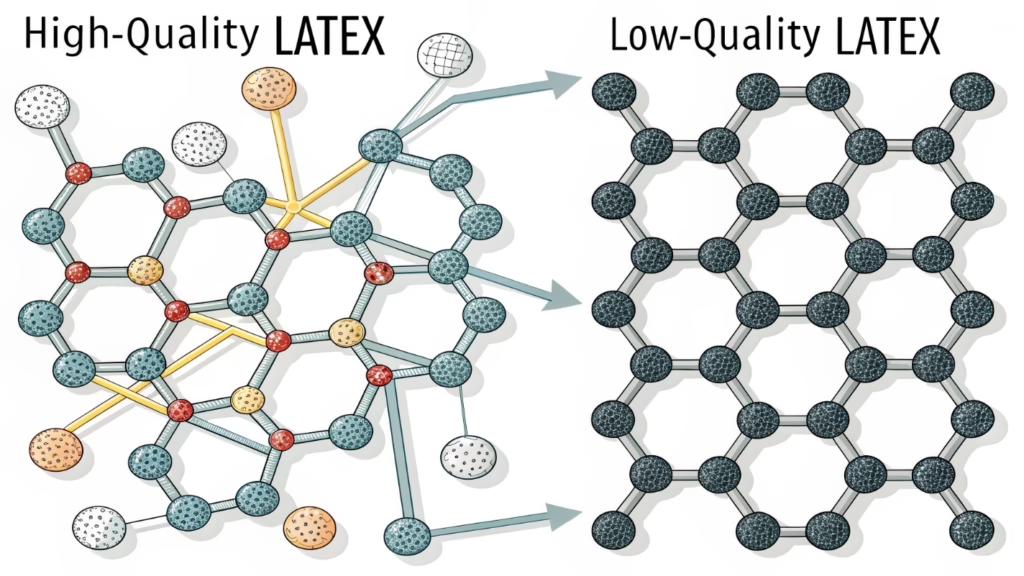
As the Vice General Manager at AIHUA BALLOON, I watch our mixing process closely. The secret to a balloon that expands without popping lies in the invisible bonds between molecules. Imagine a bowl of cooked spaghetti. If the strands are short and dry, they pull apart easily. If they are long and tied together, they stretch. That is what sulfur does to natural rubber latex during the heating process. It ties the polymer chains together.
Many factories try to cut costs by adding fillers. They use things like calcium carbonate5. This is like adding sand to the spaghetti sauce; it makes the bonds weak. When you inflate a balloon with fillers, the pressure rises, and the walls thin out. The weak spots break, and the balloon bursts.
Here is a simple way to look at the difference:
| Feature | High-Quality Latex (AIHUA) | Low-Quality Latex (Cheap Fillers) |
|---|---|---|
| Molecule Chain | Long and flexible | Short and brittle |
| Cross-linking | Strong sulfur bonds | Weak or uneven bonds |
| Inflation Limit | Expands past standard size | Bursts before full size |
| Feel | Soft and uniform | Chalky or stiff |
For a buyer, this chemistry matters. If the cross-linking is poor, the balloon cannot handle the physics of air pressure. It will fail safety standards like ISO90016. We test our batches to ensure the chemical balance is perfect. We want the balloon to stretch, not snap. This chemistry is the only thing standing between a colorful party and a loud pop.
What Physics Principles Determine Helium Retention in Foil vs. Latex Balloons?
Floating balloons dropping too soon ruins the party atmosphere. Clients blame the supplier when helium escapes too fast.
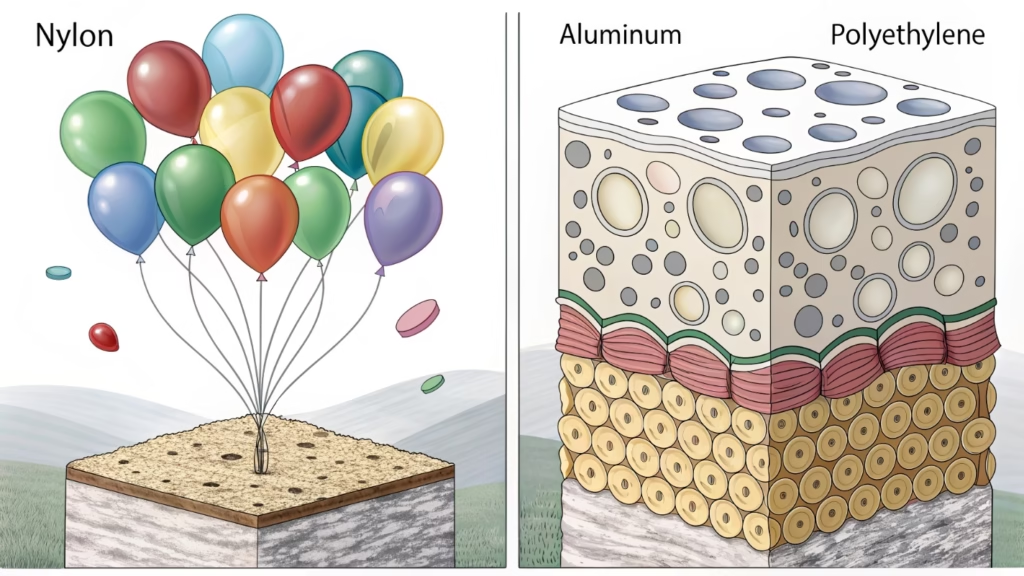
Helium atoms are very small and escape through microscopic pores in latex. Foil balloons use non-porous nylon or polyester coated with polyethylene. This barrier physics prevents diffusion7, allowing foil balloons to float for weeks, while standard latex lasts only hours without treatment.
To understand why stock choices matter, we must look at atomic physics. Helium is the second smallest element in the universe. It is an escape artist. Standard latex looks solid to the naked eye, but under a microscope, it looks like a sponge. It is full of holes. The helium atoms are smaller than these holes.
This process is called diffusion. The gas inside is under high pressure. It wants to go to the area of low pressure outside. It pushes through the latex wall. This is why a standard latex balloon drops after 12 to 24 hours.
Foil balloons are different. They rely on "barrier properties8." We make them by sandwiching layers together.
The Foil Structure
- Nylon/Polyester Base: Provides strength.
- Aluminum Layer: Provides the shiny look and blocks gas.
- Polyethylene Coating: Allows heat sealing.
The metal layer is dense. Helium atoms cannot fit through it easily. It is like trying to walk through a solid wall versus walking through a forest.
For your supply chain, this dictates your inventory mix. If your customers need displays that last for weeks in a store, latex will not work without chemical treatments like Hi-Float. Foil is the better physical choice. We ensure our foil balloons have thick enough layers to maximize this barrier effect. We want your customers to enjoy their decorations for days, not hours.
How Do Temperature and Altitude Changes Impact Balloon Inflation Stability During Transport?
Shipping inflated or sealed goods is risky; they often pop or shrink in transit. This causes loss of stock before it even reaches the shelf.
According to Charles’s Law9, gas expands when hot and contracts when cold. Higher altitude lowers outside pressure, causing internal gas to expand. If you ignore these physics during transport, balloons will burst in hot trucks or deflate in cold warehouses.
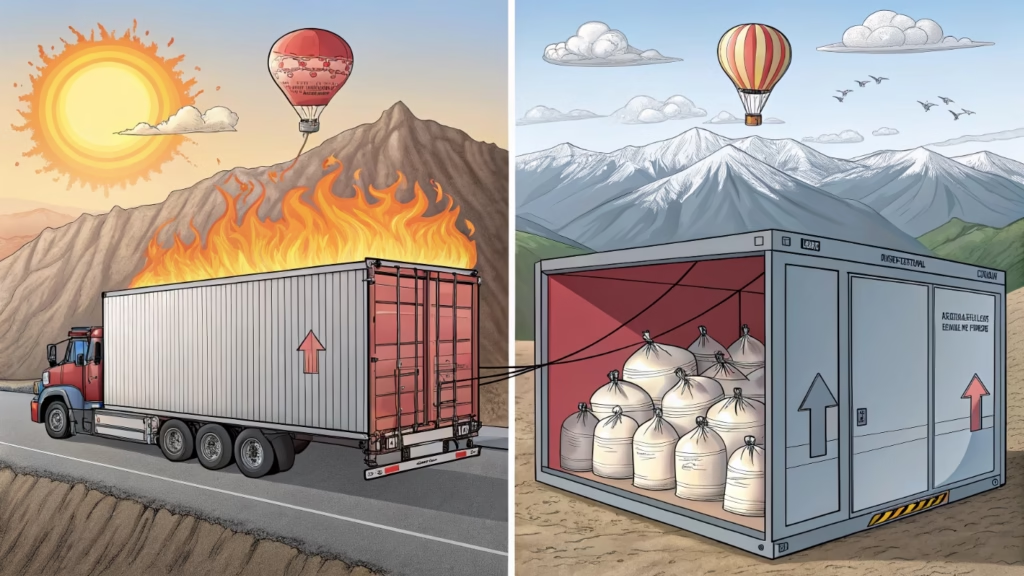
I learned this lesson early in my career. We shipped a container to a very hot region. The sun beat down on the metal shipping container all day. Inside, the temperature spiked. The air inside our packaging expanded. We faced some product loss because we did not account for the heat.
This is Charles's Law in action. It states that volume is directly proportional to temperature. If the temperature goes up, the gas takes up more space. If the balloon is already full, it has no room to grow. It bursts.
Altitude acts differently but has the same result. This is about external pressure. At sea level, the air pushes against the balloon. On a mountain, the air is thin. It pushes less. The gas inside the balloon pushes out harder. The balloon grows.
Transport Considerations
- Summer Shipping: Do not over-inflate items before shipping. Leave "slack" in the material.
- Winter Shipping: Balloons may arrive looking deflated. They are not broken. Once they reach room temperature, the gas will expand again.
- Mountain Routes: If your truck crosses a high mountain range, sealed bags might pop.
We now adjust our processes based on where the product is going. For a procurement manager, this reduces waste. We calculate the "slack" needed in the packaging. We ensure the material is thick enough to handle the stress of expansion. By respecting these laws of physics, we ensure the product arrives in the same condition it left our factory.
What Innovations in Polymer Science Are Improving Wholesale Balloon Durability?
The market demands eco-friendly options that are still strong. Old biodegradable balloons were often weak and shelf-stable for short periods.
New polymer science focuses on bio-additives10 that maintain the structural integrity of latex chains while allowing faster breakdown after use. Improvements in curing agents11 also create tighter molecular bonds, increasing burst resistance without sacrificing the natural biodegradability required by global markets.

Sustainability is a huge topic in our industry right now. In the past, if you wanted a "green" balloon, you had to accept that it was weak. It would degrade on the shelf before you even sold it. That is bad business.
At AIHUA BALLOON, we are using new science to fix this. We are looking at the curing agents. These are the chemicals that start the cross-linking process I mentioned earlier. New, plant-based curing agents help us create strong bonds without adding toxic chemicals.
We are also fighting oxidation12. Oxidation is what happens when oxygen attacks the rubber. It makes the balloon brittle and dusty. We now add organic antioxidants to our latex mix.
Why this matters for durability:
- Shelf Life: The antioxidants protect the balloon while it sits in your warehouse. It stays fresh longer.
- Performance: The new curing agents create a more uniform wall. This means fewer weak spots.
- End of Life: Once the balloon is used and exposed to bacteria in the soil, the bio-additives help it break down faster.
This is a balance of chemistry. We use critical thinking to adjust the formula. We want the balloon to be tough like plastic while it is being used, but degrade like a leaf when it is thrown away. This innovation protects your brand image. You can sell a product that is safe, strong, and responsible. It proves that we do not have to choose between quality and the environment.
Conclusion
Understanding physics and chemistry guarantees better balloon quality. From latex cross-linking to gas laws, science ensures safety and profit. We apply these rules to make your moments colorful.
-
Explore the science of balloon inflation to understand how internal pressure affects performance and longevity. ↩
-
Learn about Boyle's Law to grasp the fundamental principles of gas behavior in balloons. ↩
-
Understand the vulcanization process to see how it enhances the strength and flexibility of latex. ↩
-
Explore the role of cross-links in polymer chemistry to appreciate their impact on balloon performance. ↩
-
Learn about the impact of fillers like calcium carbonate on balloon integrity and performance. ↩
-
Understand ISO9001 standards to ensure your balloon products meet safety and quality requirements. ↩
-
Explore diffusion to understand how gas escapes from balloons and impacts their float time. ↩
-
Learn about barrier properties to see how they enhance the longevity of foil balloons. ↩
-
Understand Charles's Law to prevent balloon damage during shipping and transport. ↩
-
Discover how bio-additives enhance balloon durability while promoting environmental responsibility. ↩
-
Understand the importance of curing agents in creating strong, durable balloons. ↩
-
Learn about oxidation to understand how it impacts the longevity and quality of balloons. ↩
